Suturion secures 24 million for the upcoming launch
The Lund based MedTech company Suturion has developed a medical instrument designed to reduce complications and enhance patient safety following open abdominal surgery. The company is now preparing for an international launch by securing SEK 24 million in a recently concluded investment round.
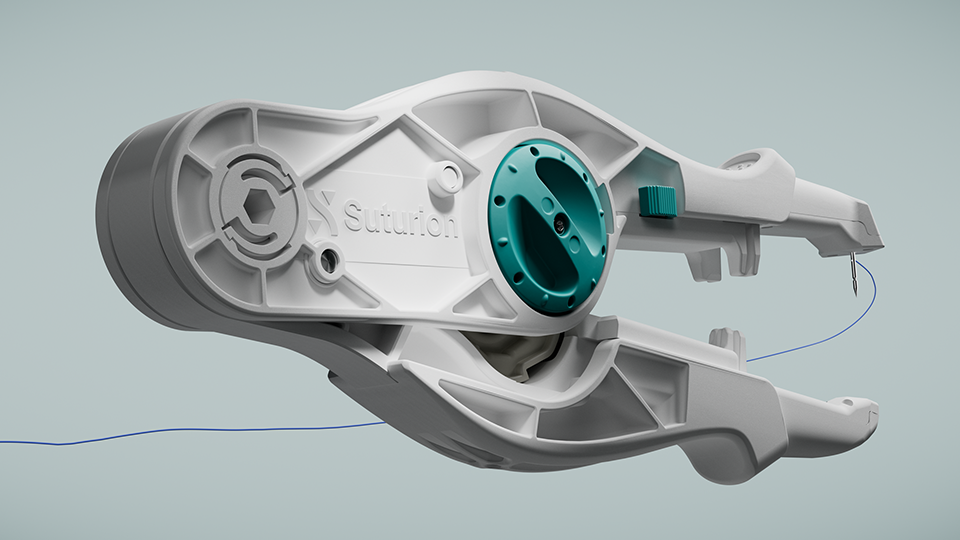
Suturion aims to address a global healthcare challenge: one in three patients undergoing open abdominal surgery suffer painful complications, often requiring additional surgery. Approximately 30 million open abdominal surgeries are performed globally each year. The innovative tool, Suture-TOOL, resembling an advanced sewing machine, is designed to close the abdomen with higher quality – thereby increasing patient safety.
The company, founded six years ago by surgeon Gabriel Börner, is now preparing for launch on several key markets. Several new investors within the MedTech sector participated in the investment round, including Scale Up Life Science Invest and Impilo Partners, alongside existing investors. This marks the company’s third investment round.
– What a wonderful way to start the new year! We are so grateful for this show of trust from our investors, demonstrating their belief in Suturion’s potential. This gives us the best possible conditions for the upcoming launch. The investors also bring valuable experience to our journey. A crucial step toward our goal to help as many patients as possible, both in Sweden and globally, says Paan Hermansson, CEO of Suturion.
Suturion will soon finalize an extensive clinical study at Helsingborg Hospital. The results are expected to show that the use of Suture-TOOL makes it possible for surgeons to perform abdominal wall closure significantly faster and with higher quality compared to the current practice of manual suturing. Manual suturing is both time-consuming and technically challenging for the surgeon. Currently, one in three patients experiences complications such as site infections, incisional hernia and burst abdomen. Two common surgeries that Suturion will focus on are abdominal cancer surgery as well as caesarean section.
The company plans to launch in the U.S. within the next six months and was recently accepted into the prestigious Safer Technologies Program (STeP) at the U.S. Food and Drug Administration (FDA), which expedites the process toward FDA approval. Also, within the year, Suturion plans to launch in the Middle East and Europe.