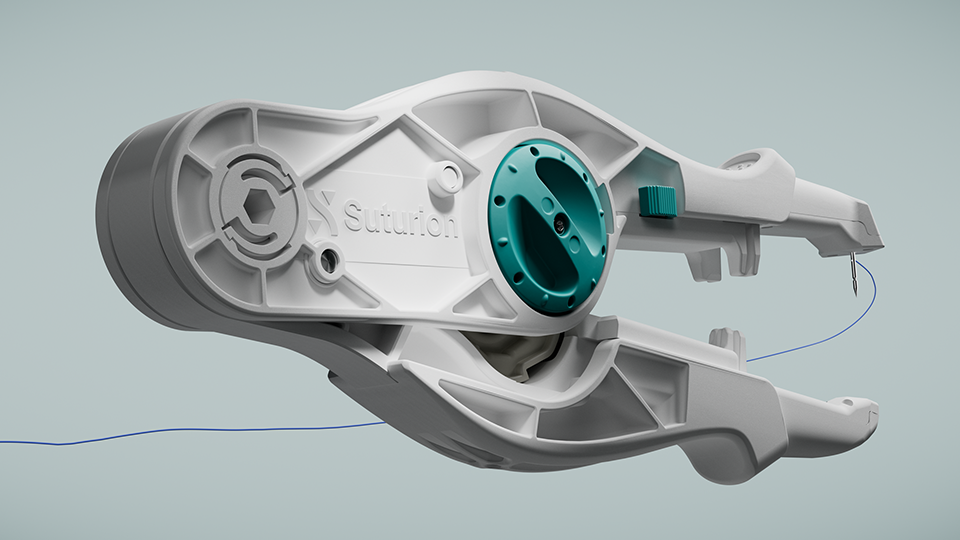
Suturion receives FDA clearance
It is now official that the U.S. Food and Drug Administration (FDA) has granted clearance for the surgical device SutureTOOL™, developed by the Swedish MedTech company Suturion. This marks a significant milestone toward the launch of this first-of-its-kind device, aiming to improve patient outcome, secure surgical precision and reduce healthcare costs.
In the fall of 2023, Swedish Suturion was one of few companies worldwide accepted into the FDA’s prestigious Safer Technologies Program (STeP). The program is designed to accelerate the approval process for products that will increase patient safety compared to alternatives on the market. SutureTOOL has now received FDA clearance, and the company is setting its sights on a U.S. launch.
– The FDA’s recognition is a milestone for Suturion and means that our device soon will be able to contribute to reducing the risk of patient complications following open abdominal surgery. The U.S. will be the first market where SutureTOOL is launched, and our aim is that it will be available already this spring, says Paan Hermansson, CEO of Suturion.
1 in 3 patients suffer painful complications
Around one in three patients experience painful complications after open abdominal surgery* that many times require additional surgeries. The current practice of manual suturing can also be both time-consuming and technically challenging for the surgeon.
SutureTOOL is a first-of-its-kind suturing device designed to increase patient safety. It can be compared to a very advanced sewing machine that allows surgeons to perform abdominal wall closure in a standardized, efficient, and safe way. By minimizing complications, it also eases the burden on the healthcare system.
U.S. launch planned for spring
The plan is to launch SutureTOOL on the U.S. market this spring 2025. Suturion is in the final stages of selecting a partner to sell and market the device in the U.S. However, the company is already present in the U.S. to initiate the purchasing process in several main hospital centers on the East Coast.
Suturion was founded 2018 in Lund, Sweden, by surgeon Gabriel Börner. Last year, the company secured an additional SEK 24 million in a funding round to prepare for the commercial launch.
*1. Deerenberg EB et al. Small bites versus large bites for closure of abdominal midline incisions (STITCH): a double-blind, multicentre, randomised controlled trial. Lancet. 2015 Sep 26;386(10000):1254-1260. doi: 10.1016/S0140-6736(15)60459-7. Epub 2015 Jul 15. PMID: 26188742.
2. HART Collaborative. Incisional hernia following colorectal cancer surgery according to suture technique: Hughes Abdominal Repair Randomized Trial (HART). Br J Surg. 2022 Sep 9;109(10):943-950. doi: 10.1093/bjs/znac198. Erratum in: Br J Surg. 2022 Dec 13;110(1):126. PMID: 35979802; PMCID: PMC10364691.